Summary of serological diagnostic methods - Part 2
The article was made by a doctor of Laboratory - Vinmec Central Park International General Hospital
There are many comparisons of serological techniques listed in this article, it is probably best to acknowledge what is possible in general, but to add any technique is very much dependent on the specific agent. and disease-causing conditions. In general, there is a trend towards standardization, automated execution of large numbers of specimens, and point-of-care testing.
6. Immunofluorescence
Indirect immunofluorescence is used in many bacteriological diagnoses, and continues to be used for many of the same purposes. The substrate used is usually agar or liquid media, the bacteria can grow on eukaryotic cells [such as chlamydiae or rickettsiae (50.51)] or a combination [sometimes used] serology for Bartonella henselae (52)]. Bacteria may or may not be associated with granulosa cells, which are mounted on glass. The substrate was added to serum dilutions in different wells on the slide, and fluorescently bound antibodies were used to detect human antibodies (Figure 3). The signal shall be read with a fluorescence microscope of suitable filter. The detected antibody can be either IgG or IgM. In the past, the constancy of substrate-coupled fluorescein was problematic, but this problem is greatly alleviated. In addition to luminescence, there are several other fluorescein agents that can be used to bind antibodies. Both the method of immobilization and the nature of the bacteria isolate can affect the quality of fluorescence. Most of the important variations are due to the observer, who must have an understanding of the luminescence intensity regardless of the scoring method or the criteria used to determine the fluorescence end- points). For this reason alone, the titer of IFA may vary according to different test sites. The best assessment is to bring to a site that has more experience in fluorescence microscopy observations than one that is not usually done.

Hình 3: Thử nghiệm miễn dịch huỳnh quang gián tiếp (IFA)
Because of the ease of IgM detection, IFA is used for rapid diagnosis, as IFA can be done within a few hours. Significant IgM titers in IFA tend to be relatively low when compared with IgG titers. More than fourfold IgG-IFA change would be as significant as the CF test. Many IgM-IFA trials are commercially available and some of them are still in use. Most of the good tests are IgM detection methods. The IgM-IFA test may be sensitive but not specific for rheumatoid factor.
The conventional IFA tests as above, use a rigid stand and are observed directly with a microscope, but the hard phase fluorescence test can also be read with an automatic reader. This is based on the FIAX system (53). Furthermore, fluorescent markers can be used in the liquid phase so that antigen-antibody interactions are better in the liquid phase than in the hard phase.
The conventional IFA tests as above, use a rigid stand and are observed directly with a microscope, but the hard phase fluorescence test can also be read with an automatic reader. This is based on the FIAX system (53). Furthermore, fluorescent markers can be used in the liquid phase so that antigen-antibody interactions are better in the liquid phase than in the hard phase.
7. Radioimmunoassay (Radioimmunoassay)
Use of a copper microradioactive marker developed from a fluorescent marker and others, and a measurement method that has the potential to enhance detection signature and sensitivity. In general, the use of radioimmunoassay on the hard phase is quite old in bacteriological serology and was superseded by EIA more than 15 years ago. Although radioisotopes are more sensitive than other methods, this may hold promise in opening a chapter related to the basis of signatures. The difficulty of evaluating 'signal-to-noise' is also important first in the development of a beneficial experiment, and manifesting 'grey' zones necessarily occurs. The use of radioisotopes and the special circumstances involved are factors that oppose this technique.
Radioisotope precipitation (54) with radiolabeled bacterial antigens is mainly used in studies to understand the humoral immune response similar to current immunoblotting, but present as an immunoblotting pattern. serodiagnosis is important although potentially used as the definitive test.
Radioisotope precipitation (54) with radiolabeled bacterial antigens is mainly used in studies to understand the humoral immune response similar to current immunoblotting, but present as an immunoblotting pattern. serodiagnosis is important although potentially used as the definitive test.
8. Solid phase immunoassays
The introduction of hard-phase immunoassays with enzyme-labeled antibodies (enzyme immunoassays; EIA) has proven to be one of the important advances in the age of serological diagnosis, and many of the tests are widely used. It is widely used in many areas of diagnostic microbiology as well as in many other medical diagnoses (not in the field of microbiology) (55-57). Variations of the EIA are numerous, but the most commonly used are the EIA, the antibody-capture EIA, and the antibody-capture double sandwich EIA (Figure 4-6).

Hình 4: Thử nghiệm EIA gián tiếp.
Many enzymes are used for EIA testing, but the most commonly used are peroxidase and alkaline phosphatase. The nature of binding an antigen or antibody to an enzyme (without affecting the enzyme activity) is that this activity causes a change in the enzyme substrate, the change in substrate color will indicate the amount of antibody present in the blood. bar. As evident from the figure above, the EIA test is simple and preferred to be used in most serological tests. This test is sensitive and may give false positive results when the antibody in the test serum is mounted on a rigid rack or component of the antigen prepared without actually binding to the specific bacterial antigen. Likewise, the direct competitive, indirect EIA test also gives false positive results due to the high concentration of antibodies of the same species in the nonspecifically conjugated sera. For these reasons, antibody capture tests were created. This test can use a marker antigen for detection or a sandwich complex for which the labeled antibody is detected.

Hình 5: Thử nghiệm EIA tóm bắt kháng thể.
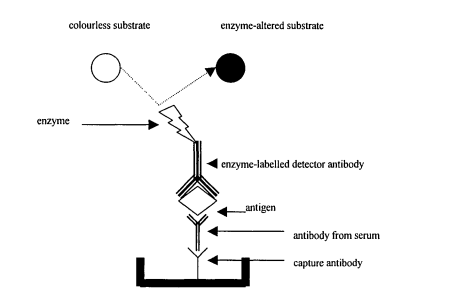
Hình 6: Thử nghiệm EIA sandwich tóm bắt kháng thể (antibody-capture double sandwich EIA)
Whether indirect or antigenic, EIA can be used to detect IgM or IgG or IgA antibodies; Furthermore, detection of IgM is valuable because it is diagnostic with only one serum specimen. EIA testing is easy to standardize, is automated, turns around quickly, and can test multiple samples at the same time. The test can be completed in 3-5 hours and some rapid methods can shorten the time from 1⁄2 hours to 1 hour.
The type of antigen used can vary from bacteria to sonicates or to pure or synthetic extracts (synthetic or recombinant peptides). These antigens can be proteins, lipids, carbohydrates, or combinations. They are mounted passively or actively on a rigid stand. Alkaline carbonate buffer (pH~9.0-9.5) enhances the binding of many antigens on conventional rigid stands. Plastics and its variants are most commonly used as mounting brackets and well configurations are suitable for washing most systems. The use of particles as a composite surface or the use of other media such as glass, nitrocellulose and nylon is also of interest. When antigen is attached to the rack, it is likely that antibodies in the patient's serum will also bind to the rack in the same way so that any possible binding sites need to be blocked with some agent. Suitable solutions for containment include buffers with albumin, gelatin, and skim milk. Efforts have been made to enhance enzyme activity by multiple mechanisms (such as amplification of the avidin-biotin marker), but most of these improvements yield only benefits in sensitivity and often come at the expense of problems. other that is usually related to specificity. However, the quality of conjugates has improved greatly over the past 2 decades and purified antibodies are also available. The use of monoclonal antibodies or polyclonal antibodies are also valuable. In some systems, enzyme markers may not be available; chemiluminescence produced by using oxidation of the resulting compound. Using automated reading systems has simplified interpretation and measurement of product (reproducible).
Although standard hard phase immunoassays include antigen and antibody classes, many commercial serological products are available that also use immunological principles, but the test time is faster with a simple one-step membrane assay. Several rapid tests are used in practice, such as serodiagnosing Helicobacter pylori (58). Both the hard phase immunoassay and the rapid membrane assay can be used in screening when large numbers of samples are available or simply because the method is simple. Disease-specific IgM appears as early as 5-7 days after illness in some infections although it is usually 7-10 days. Determination of IgG is possible in acute sera and in remission sera, however the minimal change required in EIA for a positive diagnosis may be subject to controversy. Interpretive criteria often include a 'grey zone' of suspect results based on enzyme activity measured in a continuous spectrum. Choosing a specific diagnostic threshold is also an important issue in immunoassays. Blocking assays, which compete for antibodies by using other antigen-specific antibodies, can be used for identification, although many methods are often used in situations where there is no guarantee. guarantee a positive result in a hard phase immunoassay for antigen detection. The desired antibody can also be estimated by these immunoassays, although this is not really of much value for serological diagnosis.
The nature of these immunoassays increases the maximum sensitivity to detect antibodies months to years after acute infection, even IgM. IgM can be confusing if the agent of interest is likely to re-infect.
Low factor may interfere with accurate interpretation of IgM results. Specific IgG excess also interferes with IgM determination. For this matter, interference can be ruled out by IgG-adsorbed test sera prior to IgM testing.
The type of antigen used can vary from bacteria to sonicates or to pure or synthetic extracts (synthetic or recombinant peptides). These antigens can be proteins, lipids, carbohydrates, or combinations. They are mounted passively or actively on a rigid stand. Alkaline carbonate buffer (pH~9.0-9.5) enhances the binding of many antigens on conventional rigid stands. Plastics and its variants are most commonly used as mounting brackets and well configurations are suitable for washing most systems. The use of particles as a composite surface or the use of other media such as glass, nitrocellulose and nylon is also of interest. When antigen is attached to the rack, it is likely that antibodies in the patient's serum will also bind to the rack in the same way so that any possible binding sites need to be blocked with some agent. Suitable solutions for containment include buffers with albumin, gelatin, and skim milk. Efforts have been made to enhance enzyme activity by multiple mechanisms (such as amplification of the avidin-biotin marker), but most of these improvements yield only benefits in sensitivity and often come at the expense of problems. other that is usually related to specificity. However, the quality of conjugates has improved greatly over the past 2 decades and purified antibodies are also available. The use of monoclonal antibodies or polyclonal antibodies are also valuable. In some systems, enzyme markers may not be available; chemiluminescence produced by using oxidation of the resulting compound. Using automated reading systems has simplified interpretation and measurement of product (reproducible).
Although standard hard phase immunoassays include antigen and antibody classes, many commercial serological products are available that also use immunological principles, but the test time is faster with a simple one-step membrane assay. Several rapid tests are used in practice, such as serodiagnosing Helicobacter pylori (58). Both the hard phase immunoassay and the rapid membrane assay can be used in screening when large numbers of samples are available or simply because the method is simple. Disease-specific IgM appears as early as 5-7 days after illness in some infections although it is usually 7-10 days. Determination of IgG is possible in acute sera and in remission sera, however the minimal change required in EIA for a positive diagnosis may be subject to controversy. Interpretive criteria often include a 'grey zone' of suspect results based on enzyme activity measured in a continuous spectrum. Choosing a specific diagnostic threshold is also an important issue in immunoassays. Blocking assays, which compete for antibodies by using other antigen-specific antibodies, can be used for identification, although many methods are often used in situations where there is no guarantee. guarantee a positive result in a hard phase immunoassay for antigen detection. The desired antibody can also be estimated by these immunoassays, although this is not really of much value for serological diagnosis.
The nature of these immunoassays increases the maximum sensitivity to detect antibodies months to years after acute infection, even IgM. IgM can be confusing if the agent of interest is likely to re-infect.
Low factor may interfere with accurate interpretation of IgM results. Specific IgG excess also interferes with IgM determination. For this matter, interference can be ruled out by IgG-adsorbed test sera prior to IgM testing.
9. Liquid phase immunoassays
Of course, using the hard phase is easy for antigen-antibody interactions as detailed above, however the liquid phase will be suitable for many confirmatory assays (59). Essentially, a change in enzyme activity or fluorescence can increase antigen and antibody binding and change marker activity. The test can be formulated as a one-step test and the autoreader can be used to standardize the quantification. Since the reaction is carried out in the liquid phase, the nature of the antigen allows the immune complex to remain suspended and then this form is beneficial because the antigen is smaller, such as bacterial components, enzymes or bacterial toxins. , rather than whole bacteria or large complexes. One method, for example, is affected by the level of antibiotics, like aminoglycosides. Immunoassays in liquid phase are also known as homogeneous tests.
10. lmmunoblotting
In fact, the conventional immunoblotting (often referred to as Western blotting) for serology is different from the hard phase EIA variants (60–62). The big difference is that the titres of the wells in the EIA on the hard phase are usually measured with a colorimetric spectrophotometer, which indirectly indicates the presence of antibodies, whereas the antibody response to a specific antigen can be seen in immunoblot test separates bacterial antigens.
Most antigens in the immunoblotting assay are expressed as sodium dodecyl sulphate polyacrylamide reduction lines on gel electrophoresis (SDS-PAGE) (63). They arrange according to the molecular weight change from highest to lowest along the way. Although some antigens may appear to have a certain migration rate related to their molecular weight, they may actually have molecular weight differences due to the electrophoretic conditions and the structure of the antigen. The resolution pattern of antigens moved on a rigid stand will represent the antibody in the serum and bind the enzyme to the detectable antibody. The stiffeners can be nylon, nitrocellulose (most commonly), and cellulose derivatives. Most of the resolving structures in conventional SDS-PAGE are whole or partial polypeptides. However, it is possible that the pattern of resolution of glycolipids using another technique and followed by immunoblotting has been rarely investigated. It must be recognized, however, that the antigens identified by immunoblotting are preselected by the process that selects resolution and the size or nature of the antigen may be expressed differently.
Immunoblotting can perform selectivity for IgM, IgG or IgA antibodies using appropriate conjugated. The nature of the test is more likely to provide a method of serological determination than other methods that use indirect indicators of antigen-antibody interactions. This is best illustrated by the use of immunoblotting in Lyme disease (chapter 25). The same response view for a definitive diagnosis may include one or more antigens. There is considerable variation in the humoral response to most bacteria and much heterogeneity can be seen with immunoblotting where multiple antigenic responses are identified at once. Conversely, there may be some dominant antigens that are commonly recognised, while many others may be the subject of inconsistent recognition. Furthermore, many identifiers are not absolute negative or positive when positive graduations can be seen for any given antigen (64). Indeed, the details of the method can make a big impact on immunoblotting results. Also, some run-to-run variation in antibody response patterns can be seen (it is possible to see some run-to-run variation in antibody response patterns). Some antigens may be common in many bacteria and must therefore be considered with caution as serological markers, such as flagellate proteins of borreliae and treponemes (65). Although a dominant antigen can be seen, the specificity of the response pattern seems to be greater if more than one antigen is used in the positive criterion.
During the 1980s, standardization of immunoblots was very difficult (66), but many improvements have been made in this area, such as the invention of a commercial immunoblots test that gives reliable results. The interpretation of the diagnostic positive immunoblot situation for all parts of the world must be carefully considered so that it can be assured that the antigenic response will be the same when conventional antigenic substrates are used. . In Lyme disease (see chapter 25), for example, there are some significant structural differences between the North American strain and the European strain.
One difficulty of immunoblotting, even when the substrates are commercially available, requires that the method can be used on a small number of specimens and is more commonly used for identification purposes than testing on a large batch. specimens.
The production of polypeptides from protozoa, which can damage other proteins from the growth medium, presents a theoretical advantage in increasing the chance of including nonspecific antigens. In this regard, it is promising in the future to develop purified and recombinant antigens that can solve this situation (67).
Immunoblotting can be a very sensitive technique compared to the EIA method. Since all antibodies are lost after several months of infection, variation in immunoblot patterns can vary as less activity than those antigens that normally do not appear first (68).
Invite you to follow the document on Serological Diagnosis of Infections by Doctor Tran Thi Ngoc Anh including:
Serological Diagnosis of Infections Antigens and antigenic variation Antigen relationship Voidness specificity of the humoral immune response Summary of serodiagnostic methods - Part 1 Summary of serodiagnostic methods - Part 2 General aspects of utilization in hematology Bar The Complicated Situation in Serological Diagnosis Source: Nevio Cimolai
Children's and Women's Health Center of British Columbia, Vancouver, British Columbia, Canada
Most antigens in the immunoblotting assay are expressed as sodium dodecyl sulphate polyacrylamide reduction lines on gel electrophoresis (SDS-PAGE) (63). They arrange according to the molecular weight change from highest to lowest along the way. Although some antigens may appear to have a certain migration rate related to their molecular weight, they may actually have molecular weight differences due to the electrophoretic conditions and the structure of the antigen. The resolution pattern of antigens moved on a rigid stand will represent the antibody in the serum and bind the enzyme to the detectable antibody. The stiffeners can be nylon, nitrocellulose (most commonly), and cellulose derivatives. Most of the resolving structures in conventional SDS-PAGE are whole or partial polypeptides. However, it is possible that the pattern of resolution of glycolipids using another technique and followed by immunoblotting has been rarely investigated. It must be recognized, however, that the antigens identified by immunoblotting are preselected by the process that selects resolution and the size or nature of the antigen may be expressed differently.
Immunoblotting can perform selectivity for IgM, IgG or IgA antibodies using appropriate conjugated. The nature of the test is more likely to provide a method of serological determination than other methods that use indirect indicators of antigen-antibody interactions. This is best illustrated by the use of immunoblotting in Lyme disease (chapter 25). The same response view for a definitive diagnosis may include one or more antigens. There is considerable variation in the humoral response to most bacteria and much heterogeneity can be seen with immunoblotting where multiple antigenic responses are identified at once. Conversely, there may be some dominant antigens that are commonly recognised, while many others may be the subject of inconsistent recognition. Furthermore, many identifiers are not absolute negative or positive when positive graduations can be seen for any given antigen (64). Indeed, the details of the method can make a big impact on immunoblotting results. Also, some run-to-run variation in antibody response patterns can be seen (it is possible to see some run-to-run variation in antibody response patterns). Some antigens may be common in many bacteria and must therefore be considered with caution as serological markers, such as flagellate proteins of borreliae and treponemes (65). Although a dominant antigen can be seen, the specificity of the response pattern seems to be greater if more than one antigen is used in the positive criterion.
During the 1980s, standardization of immunoblots was very difficult (66), but many improvements have been made in this area, such as the invention of a commercial immunoblots test that gives reliable results. The interpretation of the diagnostic positive immunoblot situation for all parts of the world must be carefully considered so that it can be assured that the antigenic response will be the same when conventional antigenic substrates are used. . In Lyme disease (see chapter 25), for example, there are some significant structural differences between the North American strain and the European strain.
One difficulty of immunoblotting, even when the substrates are commercially available, requires that the method can be used on a small number of specimens and is more commonly used for identification purposes than testing on a large batch. specimens.
The production of polypeptides from protozoa, which can damage other proteins from the growth medium, presents a theoretical advantage in increasing the chance of including nonspecific antigens. In this regard, it is promising in the future to develop purified and recombinant antigens that can solve this situation (67).
Immunoblotting can be a very sensitive technique compared to the EIA method. Since all antibodies are lost after several months of infection, variation in immunoblot patterns can vary as less activity than those antigens that normally do not appear first (68).
Invite you to follow the document on Serological Diagnosis of Infections by Doctor Tran Thi Ngoc Anh including:
Serological Diagnosis of Infections Antigens and antigenic variation Antigen relationship Voidness specificity of the humoral immune response Summary of serodiagnostic methods - Part 1 Summary of serodiagnostic methods - Part 2 General aspects of utilization in hematology Bar The Complicated Situation in Serological Diagnosis Source: Nevio Cimolai
Children's and Women's Health Center of British Columbia, Vancouver, British Columbia, Canada
Bài viết này được viết cho người đọc tại Sài Gòn, Hà Nội, Hồ Chí Minh, Phú Quốc, Nha Trang, Hạ Long, Hải Phòng, Đà Nẵng.






