This article was written by Phạm Thị Kim Dung, MSc, BPharm, Department of Pharmacy - Vinmec Times City International Hospital
The design of the dosage form is a crucial step that determines the quality of the drug form. When designing the dosage form, it is necessary to consider the correlation between the components in the dosage form under the direct impact of the formulation technique to find the optimal solution for each product.
1. What is a dosage form?
The concept of a dosage form is defined as follows:
A dosage form is the final pharmaceutical product obtained through formulation, where the active ingredient is combined with excipients and presented in a specific form to ensure safety, efficacy, patient convenience, ease of storage, and cost-effectiveness.
For example, Chloramphenicol is a bitter-tasting active ingredient that is difficult to swallow. It is formulated into tablets, hard capsules, or suspensions to limit the bitterness, making it easier for patients to take the medicine and improving the therapeutic effectiveness of the drug.
A dosage form includes: Active ingredient and Excipients + Packaging
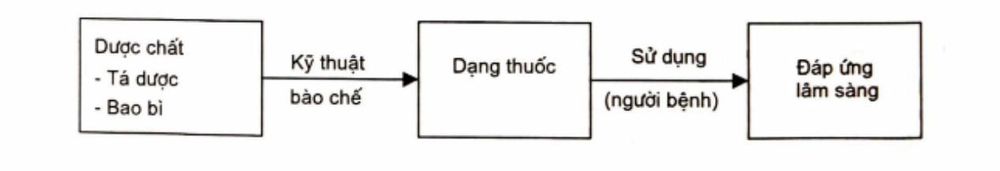
2. Why does a drug have so many different dosage forms?
To maximize the therapeutic effect of the active ingredient, dosage forms are designed considering the three key components mentioned above.. Additionally, based on research on influencing factors, the formulator decides on the dosage form. These factors include:
- Factors affecting the release and absorption process of the active ingredient in the patient’s body, such as the route of administration, age, disease condition, etc.
For example, pain relievers and antipyretics containing paracetamol have different dosage forms to suit various patient groups: Syrup form, effervescent powder form, rectal suppository form for pediatric, neonatal, or patients who have difficulty swallowing tablets. Injectable forms for patients needing rapid pain relief and fever reduction and severe conditions where oral administration is not possible. Effervescent tablets dissolve rapidly in water, leading to faster absorption and quicker onset of action compared to conventional tablets.
- The physicochemical properties of the active ingredient require the selection of suitable excipients, formulation techniques, and packaging to optimize the dosage form’s effectiveness..

Tetracycline hydrochloride, if compressed with dicalcium phosphate excipients, will have reduced effectiveness when taken due to the formation of a poorly soluble complex with dicalcium phosphate, leading to reduced drug absorption.
Alkaline glass containers can precipitate the active ingredient, which is an alkaloid salt, in injectable drugs. Some impurities in plastic containers for eye drop solutions can increase the degradation process of the active ingredient in the solution.
According to modern formulation science, the design of the dosage form is a crucial step that determines the quality of the drug form. When designing the dosage form, it is necessary to consider the correlation between the components in the dosage form under the direct impact of the formulation technique to find the optimal solution for each product. Formulation techniques are constantly being innovated and perfected to maximize the effect of the active ingredient in the body and create new dosage forms with high therapeutic efficacy.
3. What are the dosage forms of drugs?
Dosage forms of drugs can be classified in various ways:

3.1 Common classification by physical state of the drug
Liquid drugs: Syrups, solutions, suspensions, liquid extracts, etc. Some common forms include:
- Drug solutions: A homogeneous liquid dosage form that does not separate into layers. This form is usually absorbed faster than solid forms and causes less irritation to the gastrointestinal mucosa. However, it is more prone to bacterial contamination and is not suitable for active ingredients that are easily hydrolyzed.
- Drug suspensions: A form in which solid active ingredients are dispersed in a solvent, with the particle size usually larger than 1 micrometer. This form often requires additional excipients like surfactants and suspending agents to evenly disperse the active ingredient in the solvent. The drug should be shaken before use to ensure accurate dosing.
- Drug emulsions: A form in which liquid active ingredients are dispersed in a solvent, with particle sizes ranging from 0.1 to 100 micrometers. This form often includes emulsifying agents to evenly disperse the two phases of the drug. Other excipients like buffers, antioxidants, and preservatives may also be included.
- Medicinal syrups: Active ingredients dispersed in a sugar solution or a sweetener substitute. Typically, medicinal syrups contain 60-80% sugar in the solution.
Semi-solid drugs: Ointments, soft extracts, etc., used for application on the skin or mucous membranes of the body.
Solid drugs: Tablets, hard/soft capsules, powders, granules, etc. Some common forms include:
Tablets: Can contain one or more active ingredients along with excipients. There are chewable tablets and non-chewable tablets, including those specially formulated for extended or programmed release. Tablets generally provide more stable active ingredients compared to liquid forms but may be difficult to swallow for some groups like children and the elderly.
Capsules: A form where active ingredients and excipients are enclosed in a capsule (usually made of gelatin). Capsules are suitable for active ingredients that need to mask unpleasant odors or tastes or need protection from light.
Powders: A solid form consisting of a mixture of powdered active ingredients and excipients. This form is more stable for active ingredients than liquid forms and dissolves better than tablets, but it is harder to mask unpleasant tastes compared to tablets.
Extended or programmed release forms: Specially formulated to release the active ingredient steadily into the bloodstream at a certain concentration, reducing the frequency of dosing for the patient. These forms are often abbreviated as SR (sustained release), SA (sustained action), ER, XR, XL (extended release), TR (timed release), CR (controlled release), MR (modified release), etc.
3.2 By route of administration (common classification)
The route of administration significantly affects the drug’s action. An active ingredient introduced into the body through different routes can cause different pharmacological effects. For example, magnesium sulfate taken orally acts as a choleretic and laxative, while when injected, it acts as an anti-edema agent.
Drugs administered via the gastrointestinal tract often face absorption issues due to various factors such as digestive pH, enzymes, food, first-pass metabolism in the liver, and the drug’s transit time.
- Injectable drugs:
There are various types of injections: intramuscular, intravenous drip or infusion, subcutaneous injection.
- Drugs administered via the gastrointestinal tract:
Oral, sublingual, or chewable drugs (active ingredients are absorbed or act mainly in the small intestine), suppositories, and enemas (acting locally or absorbed through the capillary system in the rectum).
- Drugs administered via the respiratory tract:
Forms for inhalation, nebulization, nasal drops, etc.
These forms can act locally on the respiratory mucosa or have systemic effects.
- Drugs administered via the skin
Ointments, lotions, patches, powders, sprays, transdermal therapeutic systems, etc.
Most of these drugs act locally (treating itching, protecting the skin, etc.), but in some cases, the active ingredient is absorbed through the skin to have systemic effects (e.g., anti-angina, anti-motion sickness).
Additionally, based on the origin of the drug formula, dosage forms can be classified into two types:
- Formulated drugs: Prepared according to official formulas specified in authoritative documents such as Pharmacopoeias, Formularies, National Formulas, etc. Pharmaceutical companies must adhere to the exact formula and quality standards when producing these preparations.
- Compounded drugs: To enhance therapeutic efficacy, doctors prescribe specific formulations regarding dosage, concentration, and preparation method, tailored to the patient’s changing physiological and pathological conditions. Pharmacists must review the prescription, check the dosage, and consider drug interactions (paying attention to incompatibilities) and dosage form before compounding the drug.
To arrange an appointment, please call HOTLINE or make your reservation directly HERE. You may also download the MyVinmec app to schedule appointments faster and manage your reservations more conveniently.
Articles refer to the source:
- Preparation and Biopharmaceutical Techniques of Drugs – Volume 1; Department of Pharmacology, Hanoi University of Pharmacy 2006
- Tulane University School of Medicine, Medical Pharmacology, TMedWeb
To arrange an appointment, please call HOTLINE or make your reservation directly HERE. You may also download the MyVinmec app to schedule appointments faster and manage your reservations more conveniently.