This article is authored by Pharmacist Pham Thi Kim Dung - Department of Pharmacy - Vinmec Times City International General Hospital
Profertil is a medication with the active ingredient Clomiphene Citrate. It is used according to the indications provided by a physician.
1. Indications for Profertil
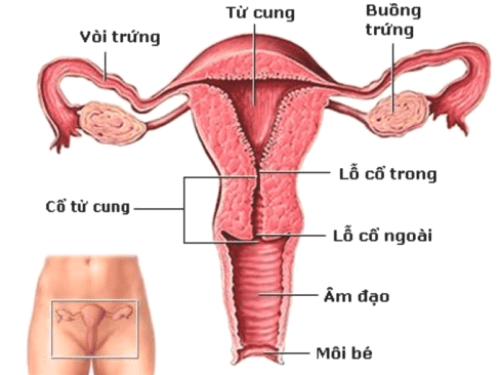
• Treatment of infertility in women with amenorrhea.
• Stein-Leventhal Syndrome or dysfunctional uterine bleeding due to ovulatory disturbances.
• Stimulation of spermatogenesis in males with infertility due to oligospermia.
2. Mechanism of Action of Clomiphene Citrate (Profertil)
• Clomiphene citrate acts as an antagonist to estrogen by competing with endogenous hormones at various sites such as the hypothalamus, pituitary gland, ovaries, endometrium, vagina, and cervix, thus inhibiting the effects of endogenous estrogen. This initiation prompts a cascade of endocrine reactions, beginning with an enhanced release of gonadotropins (LH and FSH) from the pituitary gland, leading to the formation and development of ovarian follicles.
• Clomiphene citrate increases levels of LH, FSH, and testosterone in both normozoospermic males and those with low sperm counts. There is insufficient evidence to indicate that Profertil impacts other hormones such as progesterone or androgens. Therefore, the medication is beneficial in treating infertility in women with ovulatory impairment or men with reduced sperm count.

3. Contraindications for Clomiphene Citrate (Profertil)
• Patients with impaired liver function.
• Pregnant women or those with ovarian cysts.
• Patients exhibiting dysfunction of the pituitary gland, ovaries, or other reproductive organs.
4. Adverse Effects of Clomiphene Citrate (Profertil)
• The medication may cause ovarian overstimulation, leading to the formation of ovarian cysts when administered at high doses of 100-200 mg daily for 2-3 weeks. Reducing the dosage to 50 mg daily can mitigate these undesirable effects without impairing treatment efficacy.
• Other potential adverse effects include hot flashes, gastrointestinal disturbances, and skin rashes in patients sensitive to the drug. Visual disturbances, headache, and insomnia may also occur.
• Due to the possibility of visual impairment, patients are advised against driving or operating heavy machinery while under treatment.
• As sufficient safety data for use in pregnant or lactating women is lacking, it is imperative to initiate each treatment cycle of Clomiphene only after confirming that the patient is not pregnant. This medication should not be administered to breastfeeding women.
5. Dosage and Administration of Clomiphene Citrate (Profertil)
• For the treatment of infertility due to anovulation:
1 tablet/day for 5 days, commencing on the fifth day of the menstrual cycle or any day if the patient is amenorrheic.
Ovulation typically occurs within 6-10 days following the last dose of Profertil. If no ovulation is observed after the first treatment cycle, the regimen may continue with 1 tablet/day for 5 additional days. If pregnancy does not occur after 6 treatment cycles, the likelihood of success diminishes.
• For treatment of male oligospermia:
1 tablet/day for 40-90 days. Sperm counts may increase within 4-5 weeks following the initiation of treatment.

References:
1. Profertil package insert by the manufacturer.
2. DailyMed: Monograph: CLOMIPHENE CITRATE tablet.
3. Micromedex: Clomiphene monograph.
To arrange an appointment, please call HOTLINE or make your reservation directly HERE. You may also download the MyVinmec app to schedule appointments faster and manage your reservations more conveniently.